Cannabidiol for paediatric epilepsy
Cannabidiol (CBD, Epidyolex®) in children
Cannabidiol (CBD) is a purified cannabinoid (not psychoactive). It is available in UK as Epidyolex® oral solution.
Cannabidiol (CBD, Epidyolex®) is now part of the toolbox for severe childhood epilepsies.
But it’s not “any CBD oil” — here’s what doctors and medical students should know:
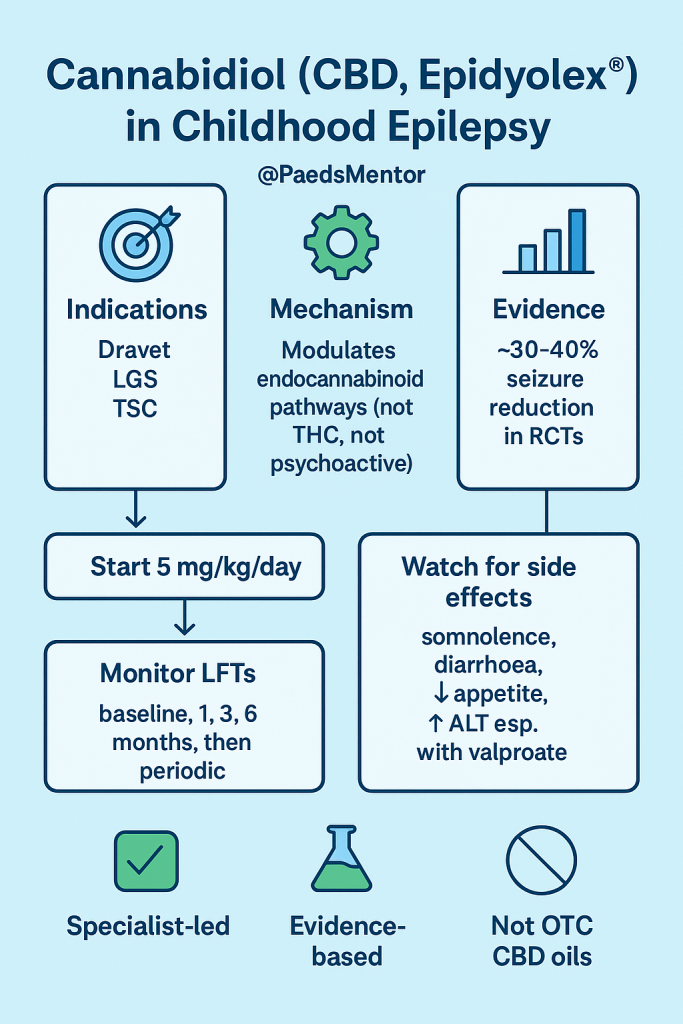
Indications (UK licence):
Dravet syndrome
Lennox–Gastaut syndrome
Tuberous sclerosis complex
Used as adjunctive therapy in children ≥2y, after other AEDs have failed.
RCTs describe ~30–40% reduction in seizure frequency.
Mechanism of Action
Not fully understood. Likely involves modulation of endocannabinoid system, TRPV1 channels, and GPR55 receptors.
Importantly: does not act like THC (no psychotropic effect).
Usual dose: Starting dose is 5 mg/kg/day BD and titrate to 10–20 mg/kg/day BD.
Monitoring is essential due to risks of hepatocellular injury, especially when combined with valproate.
Adverse Effects
Common: somnolence, diarrhoea, decreased appetite, weight loss, fatigue.
Important: hepatocellular injury (↑ ALT/AST, esp. with sodium valproate).
Sedation risk ↑ with clobazam (due to norclobazam accumulation).
Hepatic monitoring recommendations
Baseline LFTs (ALT, AST, bilirubin, ALP, GGT) before starting. Undertake full medication review (esp. valproate, clobazam). Discuss with parents about possible liver enzyme changes and need for regular bloods
Repeat at 1, 3, and 6 months, and then periodically, or more often if clinically indicated.
Thresholds (per product info and guidelines):
If ALT/AST >3× ULN with symptoms of liver injury → stop CBD.
If ALT/AST >5× ULN regardless of symptoms → stop CBD.
Mild isolated rises (<3× ULN, asymptomatic) → usually monitor closely, consider dose adjustment if rising.
