Blood Gas Analysis in children
Introduction: Why do we use Blood Gases in children?
A blood gas is a rapid and powerful diagnostic tool used to evaluate a child’s respiratory, circulatory, and metabolic status. It provides a real-time snapshot of the body’s acid-base balance, ventilation, and oxygenation. Understanding blood gases is critical for managing critically ill children.
Key Components and What They Tell Us
A blood gas result provides several key values that must be interpreted together.
pH (Potential of Hydrogen): The most important value. It indicates the overall acidity or alkalinity of the blood.
Normal Range: 7.35–7.45.
Acidosis (pH<7.35): Too much acid.
Alkalosis (pH>7.45): Too much alkali.
PaCO2 (Partial Pressure of Carbon Dioxide): This is the respiratory component and reflects how well the lungs are ventilating.
Normal Range: 35–45 mmHg.
High PaCO2 (e.g., > 45 mmHg) indicates hypoventilation (not breathing off enough CO2), leading to respiratory acidosis. This can be caused by conditions like respiratory failure, airway obstruction, or central nervous system depression.
Low PaCO2 (e.g., < 35 mmHg) indicates hyperventilation (breathing off too much CO2), leading to respiratory alkalosis. This is often a compensatory mechanism for metabolic acidosis or due to anxiety, pain, or fever.
HCO3− (Bicarbonate): This is the metabolic component and reflects the base level in the blood, primarily regulated by the kidneys.
Normal Range: 22–26 mEq/L.
Low HCO3− (e.g., < 22 mEq/L) indicates a metabolic acidosis. Common causes in children include septic shock, diabetic ketoacidosis, dehydration, and renal failure.
High HCO3− (e.g., > 26 mEq/L) indicates a metabolic alkalosis. This can be seen in conditions like severe vomiting (pyloric stenosis) or diuretic use.
Base Excess (BE): A measure of the total amount of base in the blood. It gives a more complete picture of the metabolic component than just bicarbonate.
Normal Range: -2 to +2 mEq/L.
A negative BE (Base Deficit) indicates metabolic acidosis.
A positive BE indicates metabolic alkalosis.
PaO2 (Partial Pressure of Oxygen): The oxygenation status of the blood. It’s most useful in an arterial sample.
Normal Range: 80–100 mmHg on room air.
Lactate: A common finding on blood gases.
Normal Range: 0.5–2.2 mmol/L.
A high lactate suggests anaerobic metabolism, often due to poor tissue perfusion (shock) or increased metabolic demand.
The Three-Step Approach to Interpretation
A systematic approach prevents errors and helps trainees think logically.
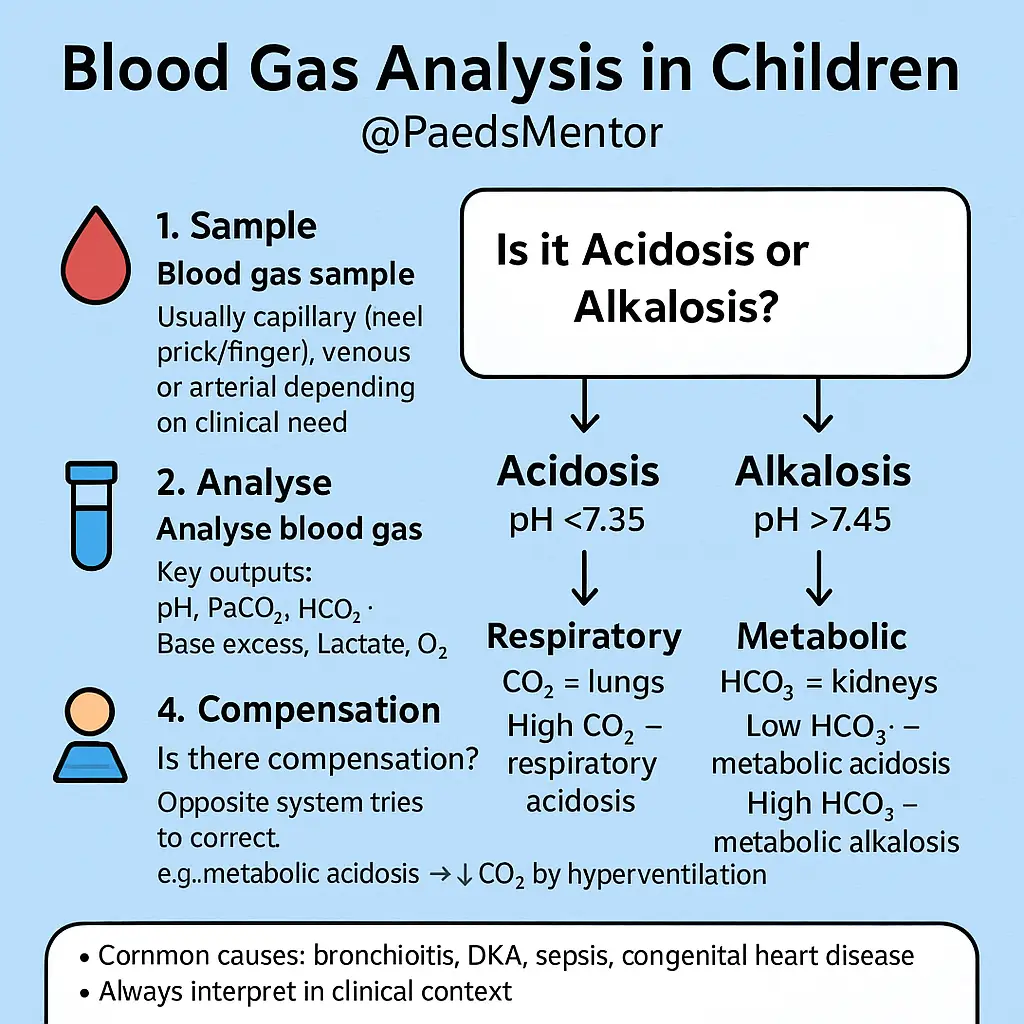
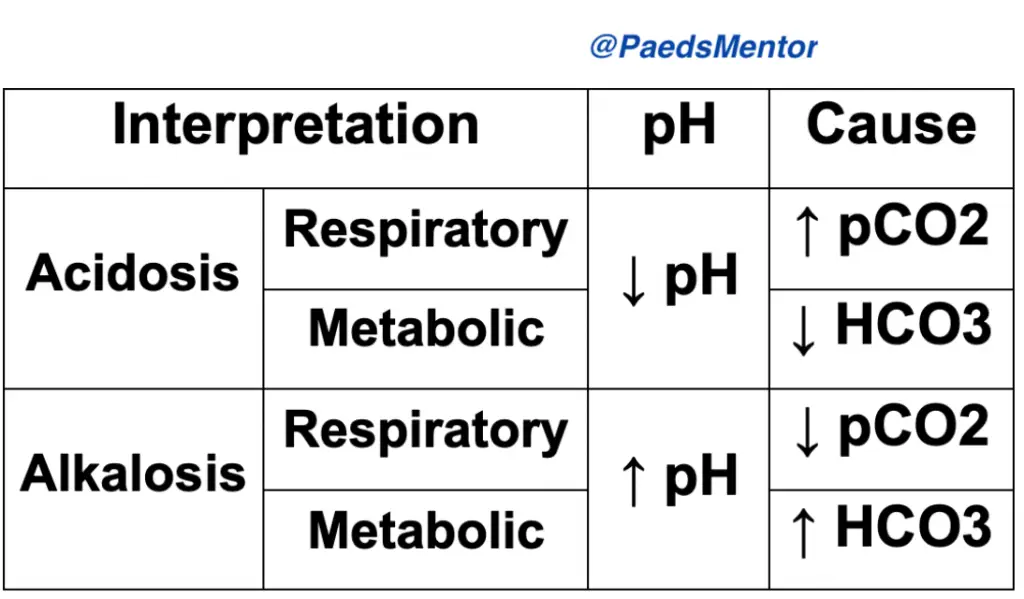
Look at the pH. Is there acidosis or alkalosis? This determines the primary problem.
Is the pH low (<7.35)? It’s an acidemia.
Is the pH high (>7.45)? It’s an alkalemia.
Examine the PaCO2 and HCO3−. Which component aligns with the pH?
If the pH is low and the PaCO2 is high, it’s a respiratory acidosis.
If the pH is high and the PaCO2 is low, it’s a respiratory alkalosis.
If the pH is low and the HCO3− is low, it’s a metabolic acidosis.
If the pH is high and the HCO3− is high, it’s a metabolic alkalosis.
Check for Compensation. Is the “other” component attempting to correct the pH?
The body’s 2 compensatory mechanisms work to return the pH to normal.
Respiratory compensation is fast (minutes to hours). For a metabolic acidosis, the body will hyperventilate to lower the PaCO2.
Metabolic compensation is slow (hours to days). For a respiratory acidosis, the kidneys will retain bicarbonate to raise the HCO3−.
Example: A child with diabetic ketoacidosis has a blood gas with pH 7.20 (low), PaCO2 30 mmHg (low), and HCO3− 15 mEq/L (low).
Step 1: The pH is low (7.20) -> Acidosis.
Step 2: The HCO3− is low. A low HCO3− causes acidosis. This is a metabolic acidosis.
Step 3: The PaCO2 is also low. This doesn’t cause acidosis; instead, the body is compensating by hyperventilating to blow off CO2 and raise the pH back towards normal. This is a compensated metabolic acidosis.
Useful Mnemonic= ROME
Respiratory component Opposite (pCO2 is in opposite direction to pH)
Metabolic component Equal (HCO3 & pCO2 is in same direction as pH)
(but it doesn’t work for mixed disorders)
Special Considerations for Children
Age-Specific Ranges: Normal values, especially for pH and PaCO2, can differ slightly in neonates and young infants compared to older children and adults. For example, neonates often have a slightly lower PaCO2 and HCO3− due to their higher respiratory rate.
Sample Type:
Arterial Blood Gas (ABG): The gold standard for assessing oxygenation (PaO2) and ventilation (PaCO2). However, obtaining an ABG in a child is painful and often not necessary.
Venous Blood Gas (VBG): Easy to obtain and useful for assessing the acid-base and metabolic status (pH, PaCO2, HCO3−, lactate). Do not use a VBG for PaO2! The venous PaO2 is significantly lower than arterial.
Capillary Blood Gas (CBG): A good compromise, often taken from the heel in infants. It gives a reasonable approximation of arterial pH and PaCO2 but is unreliable for oxygenation. Squeezing the sample too much can lead to falsely high potassium and lactate levels.
Before interpreting a blood gas, ask yourself:
- Do you know the clinical diagnosis and background information?
- Know the situation why blood gas was done
- Is the patient deteriorating or improving clinically?
- What intervention or change has happened; and what is expected on blood gas?
- How much O2 is given? Is patient’s SaO2 maintained?
- Is there a previous blood gas to compare the trend?
- Do you need to intervene / discuss this with senior doctors?
Respiratory Acidosis, common causes:
– Obstruction of airways e.g. Croup, Foreign body, Asthma
– Inadequate alveolar ventilation e.g. RDS, Bronchiolitis, Pneumonia
– Hypoventilation e.g. Neuromuscular diseases, excess opiates, severe scoliosis
– Ventilation/perfusion imbalance e.g. Collapse, Pneumothorax
Manage with CPAP, High-Flow O2, Ventilation etc
Metabolic Acidosis, common causes:
– Poor circulation causing lactic acidosis Hypotension, Sepsis, Shock, Anaphylaxis
– Excessive loss of HCO3 in the urine or gute.g. Renal Tubular Acidosis, chronic diarrhoea, Spironolactone
– Other acids in blood – DKA, Inborn error of metabolism, Ethanol, Methanol poisoning, renal failure
Manage with establishing adequate circulatory volume with fluid boluses, colloid, inotropes if necessary
Respiratory Alkalosis, common causes:
– Hyperventilation e.g. Panic attack, High altitude, Anaemia, Pulmonary oedema
– Excessive mechanical ventilation
– Drugs: Catecholamines, salicylates, doxapram, nicotine
– Hyperthermia, hepatic encephalopathy
Manage by treating the cause; e.g. for Panic attack with rebreathing in to paper bag; etc
Metabolic Alkalosis, common causes:
– Loss of acid with excessive vomiting e.g. Pyloric stenosis, Bulemia, Nasogastric free drainage
– Volume contraction with loop or thiazide diuretics,
– Congenital Adrenal Hyperplasia, Primary Aldosteronism, Renin secreting tumor, Bartter and Gitelman syndromes
